Zeolites are formed when volcanic ash and aluminosilicates of alkaline and alkaline earths either settles into lakes or water percolated through the ash beds. In either case, this chemical reaction between water and volcanic ash results in the natural formation of zeolites.
About Zeolites


Different depositional environments (temperature, location, ash/ water) present during formation explain why each natural zeolite has its own unique properties.
The clinoptilolite, a crystalline form of zeolite, is a sub-group. It is perhaps the most unique and widely used.

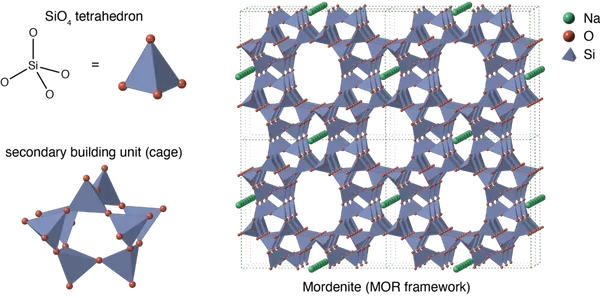
Zeolite is microporous with an open, stable, three- dimensional honeycomb structure negatively charged. The structure loosely holds Cations (negatively charged ions) that are available for exchange.
The ability to exchange one cation for another is called “Cation Exchange Capacity (CEC)”, this allows the Clinoptilolite to act as natures sponge, allowing larger molecules to be separated from smaller molecules.
